Design and Development of Cleaning Validation in Pharmaceutical Industry
Article Sidebar
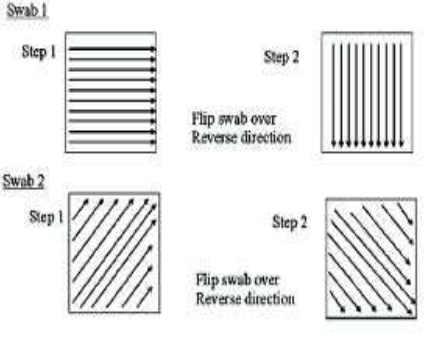
Abstract
Pharmaceutical product can be contaminated by other pharmaceutical products or Active pharmaceutical ingredients (APIs) or by cleaning agents, by microorganisms or by other materials e.g. air borne particle, dust, lubricants, raw materials, intermediates. Mainly cleaning is performed to remove product and non-product contaminating material. Ineffective cleaning can lead to contaminated product, which may be contaminated from previous product batches, cleaning agent or other extraneous material introduced into generated by the process. In pharmaceutical industry the same equipment may be used for processing different products. To avoid cross contamination source or facility configuration there is a need to ensure that cleaning procedure must strictly follow carefully established and validated method of execution.